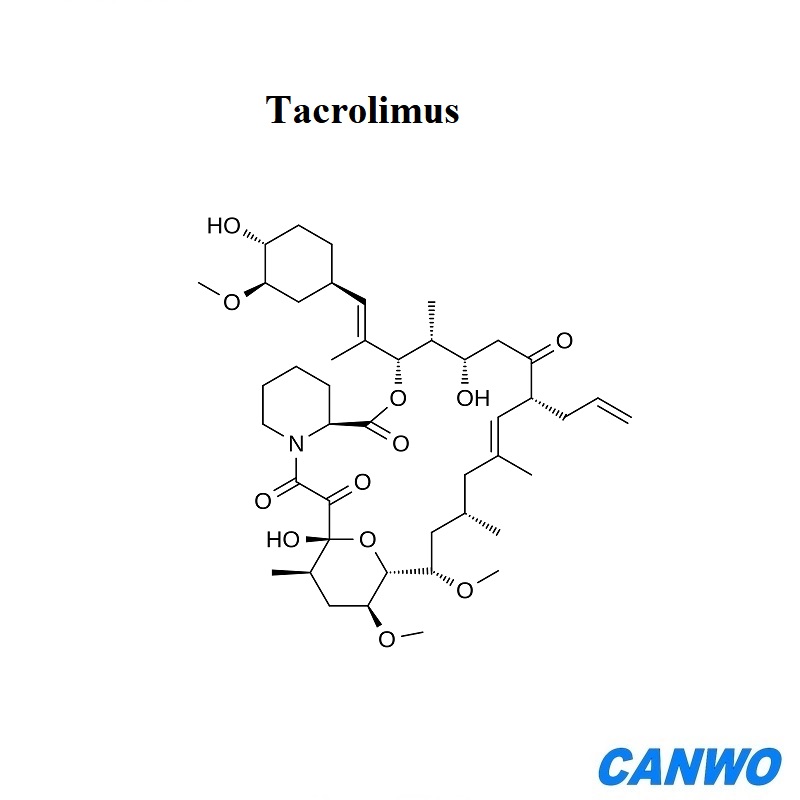
Tacrolimus
Standard: BP/USP/EP/IN HOUSE
Ceftification: GMP/CEP
MOQ: 1 kgs
Price: Please send enquiry
Tacrolimus is a is a macrolide calcineurin inhibitor immunosuppressant drug that works by decreasing the activity of the immune system. Tacrolimus can be used in different dosage forms as an agent for the prevention or treatment of certain autoimmune diseases. We supply tacrolimus raw material GMP standard with document support.
-
Standard: USP / EP / CP
-
Certification: CHINA GMP/ WC /CEP / US FDA
-
Regulatory documentation: DMF open part, Letter of Access.
Indication: (1)Tacrolimus can be used for prophylaxis of organ rejection post-transplant in combination with one or two other immunosuppressive medications. (2)Tacrolimus can be used as an agent for the prevention or treatment of certain autoimmune diseases. (3)Tacrolimus indications also include topical use in moderate to severe atopic dermatitis, as well as other off-label dermatologic disease states.
Applications:Tacrolimus raw material can be used to produce exterinal dosage such as tacrolimus oinement, cream, oral grade product such as tacrolimus capsules, tablets as well as tacrolimus injection to prevent the body from rejecting a transplanted organ.
Tacrolimus Detail Information
-
Molecular Formula:C44H69NO12
-
Molecular Weight: 804.0182
-
CAS Registry Number:104987-11-3
-
Appearance: Tacrolimus is white or almost white, crystalline powder. It is practically insoluble in water, soluble in ethanol, practically insoluble in heptane.
-
Packaging and storage:Preserve in tight, light-resistant containers, and store in a cold place.
-
Shelf life: 3 Years
-
Transport suggestion: Not Regulated. Tacrolimus is not restricted as per special provision A3.